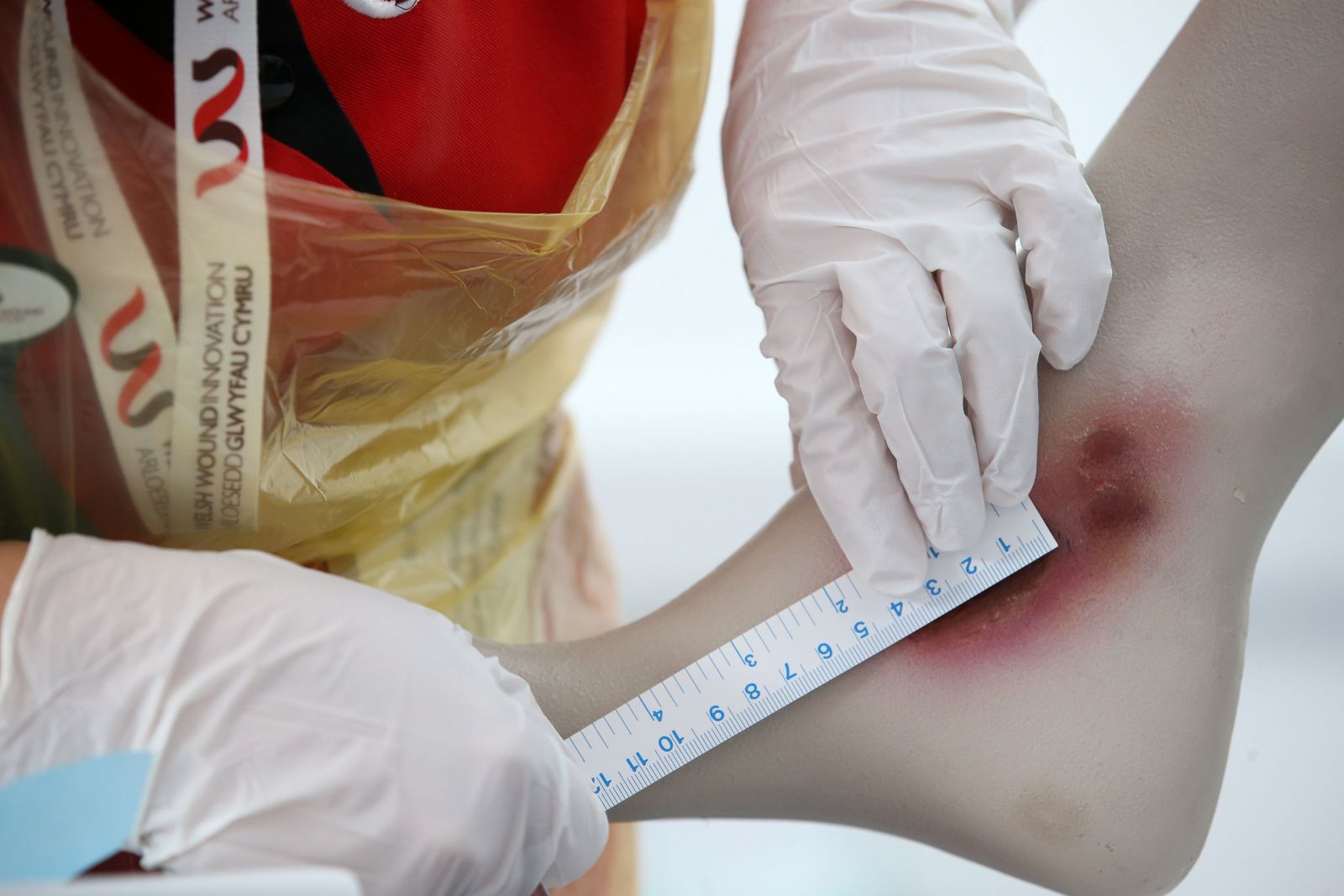
 We play a pivotal role in developing, supporting, creating and advancing wound therapies, diagnostics, and devices through protocol development and advice on study design and achievability.
We play a pivotal role in developing, supporting, creating and advancing wound therapies, diagnostics, and devices through protocol development and advice on study design and achievability. Taking a holistic view of the wound care innovation and translation journey to identify areas where support is justified and can make a difference. This involves developing a standardised process for service and product evaluations, clinical trials, and engagement activities.
Taking a holistic view of the wound care innovation and translation journey to identify areas where support is justified and can make a difference. This involves developing a standardised process for service and product evaluations, clinical trials, and engagement activities.
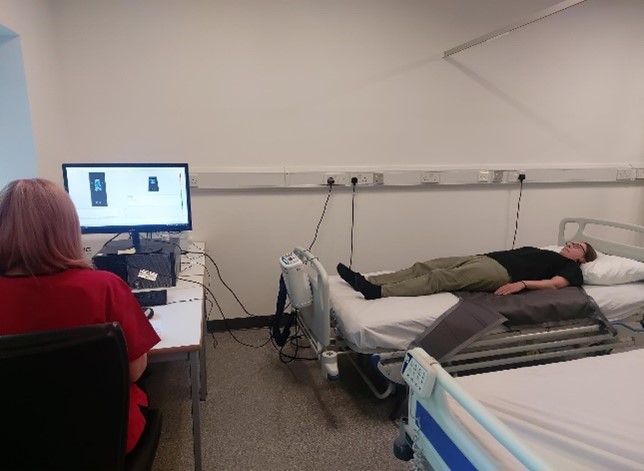
 We undertake a range of trials and projects in conjunction with commercial providers and academic institutions on a bespoke basis, tailored to specific requirements from protocol development through to reporting and publication. We are experienced in undertaking multiple stages of clinical trials investigating both medical devices and pharmaceuticals related to wound healing, from First-in-Human trials, large-scale RCT’s to Post-Market Surveillance studies.
We undertake a range of trials and projects in conjunction with commercial providers and academic institutions on a bespoke basis, tailored to specific requirements from protocol development through to reporting and publication. We are experienced in undertaking multiple stages of clinical trials investigating both medical devices and pharmaceuticals related to wound healing, from First-in-Human trials, large-scale RCT’s to Post-Market Surveillance studies. Huntleigh Healthcare Ltd. - Intermittent Pneumatic Compression Of the Thigh for the Treatment of lower limb wounds: a randomised control trial.
Huntleigh Healthcare Ltd. - Intermittent Pneumatic Compression Of the Thigh for the Treatment of lower limb wounds: a randomised control trial.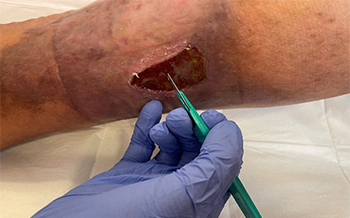 Medical Ethics Ltd. - An open, randomised, parallel group controlled single centre safety study to assess the safety and efficacy of Tri-Solfen in providing anaesthesia prior to surgical debridement of leg ulcers and post-operative pain relief (First-in-Human Study) – This study is investigating a novel product designed to reduce pain for patients pre- and post-debridement of their leg ulcers, a procedure required for wound bed management and promotion of wound healing, but which can cause pain and be difficult to tolerate for patients.
Medical Ethics Ltd. - An open, randomised, parallel group controlled single centre safety study to assess the safety and efficacy of Tri-Solfen in providing anaesthesia prior to surgical debridement of leg ulcers and post-operative pain relief (First-in-Human Study) – This study is investigating a novel product designed to reduce pain for patients pre- and post-debridement of their leg ulcers, a procedure required for wound bed management and promotion of wound healing, but which can cause pain and be difficult to tolerate for patients. FirstKind Ltd. - Microcirculatory Flux and Pulsatility in Arterial Leg Ulcers is Increased by Intermittent Neuromuscular Electrostimulation of the Common Peroneal Nerve (Non-Comparative Study) - This study found that neuromuscular electrical stimulation increased microcirculatory blood flow to the wound bed and edge in patients with ischemic lower limb wounds.
FirstKind Ltd. - Microcirculatory Flux and Pulsatility in Arterial Leg Ulcers is Increased by Intermittent Neuromuscular Electrostimulation of the Common Peroneal Nerve (Non-Comparative Study) - This study found that neuromuscular electrical stimulation increased microcirculatory blood flow to the wound bed and edge in patients with ischemic lower limb wounds. We developed from and built on the legacy of a long-standing history and involvement within wound healing research. Our record of conducting clinical research to internationally recognised standards in many formats supports our Clinical Research Organisation service as part of our offering. We are also highly experienced with the regulatory aspect of conducting clinical trials and offer project management from study conception to completion and we of course adhere to all applicable regulations and guidelines to include ICH, GCP, and ISO 14155. This ensures the scientific conduct of the clinical investigation and the credibility of the clinical investigation results while protecting the rights, safety, and well-being of all clinical trial participants.
We developed from and built on the legacy of a long-standing history and involvement within wound healing research. Our record of conducting clinical research to internationally recognised standards in many formats supports our Clinical Research Organisation service as part of our offering. We are also highly experienced with the regulatory aspect of conducting clinical trials and offer project management from study conception to completion and we of course adhere to all applicable regulations and guidelines to include ICH, GCP, and ISO 14155. This ensures the scientific conduct of the clinical investigation and the credibility of the clinical investigation results while protecting the rights, safety, and well-being of all clinical trial participants.Latest
News
Upcoming
Events
There are no events to show.
Welsh Wound Innovation Centre, Cwm Taf Morgannwg UHB, The Hub, Unit 2, Gwaun Elai, Ynysmaerdy, Llantrisant, Rhondda Cynon Taf, CF72 8XR
© 2026 WWIC
